23 Apr 2020
Abstract
The arabinosyltransferases EmbA, EmbB, and EmbC are involved in Mycobacterium tuberculosis cell wall synthesis and are recognized as the targets for the anti-tuberculosis drug ethambutol. We have determined cryo-electron microscopy and x-ray crystal structures of mycobacterial EmbA-EmbB and EmbC-EmbC complexes, in the presence of their glycosyl donor and acceptor substrates and with ethambutol. These structures show how the donor and acceptor substrates bind in the active site and how ethambutol inhibits by binding to the same site as both substrates in EmbB and EmbC. The majority of drug-resistant mutations are located nearby to the ethambutol-binding site. Collectively, our work provides a structural basis for understanding the biochemical function and inhibition of arabinosyltransferases and development of new anti-tuberculosis agents.
[Image]
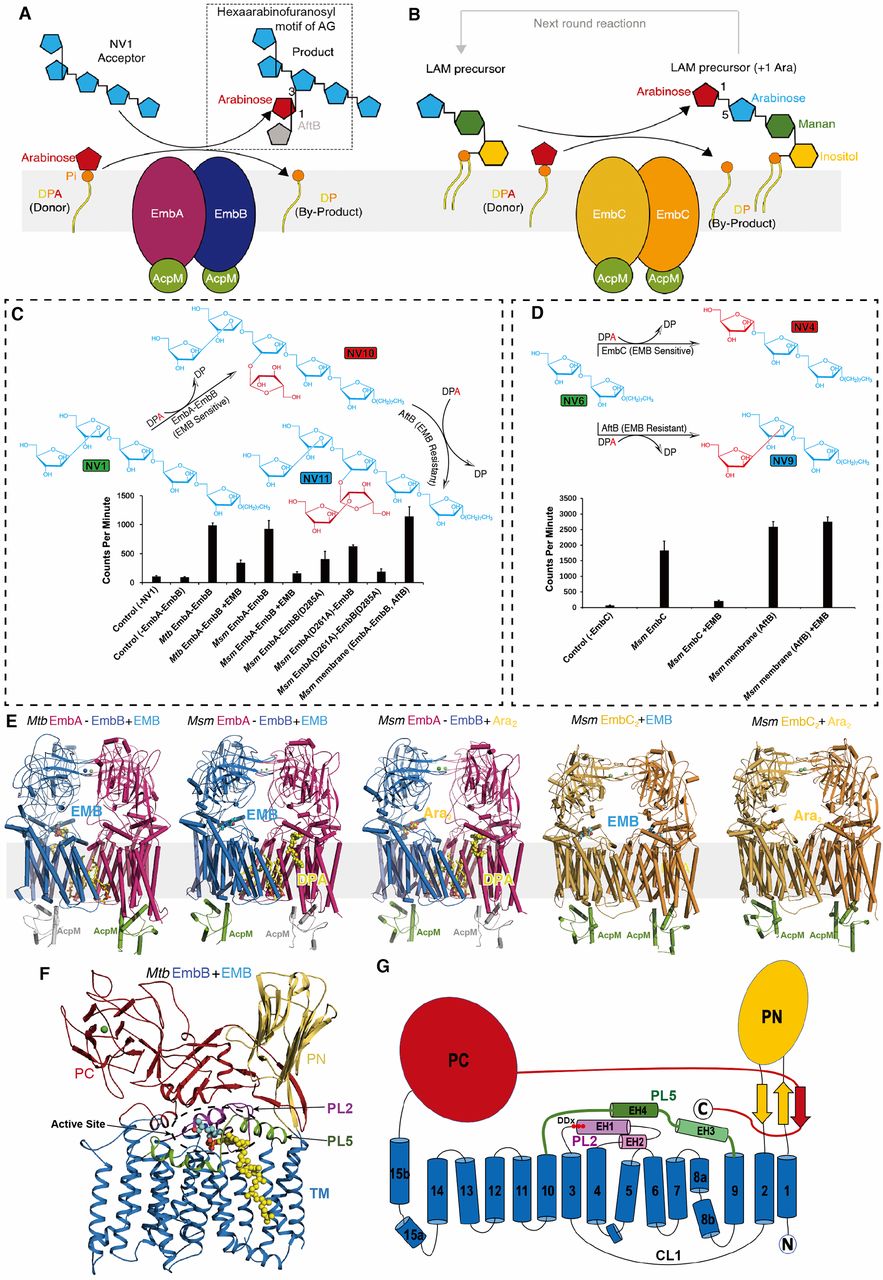
Fig. 1 Activity and overall structure of EmbA-EmbB-AcpM2 and EmbC2-AcpM2 complexes.
(A) Schematic representation of the enzyme reaction catalyzed by the EmbA-EmbB-AcpM2 complex, which transfers an arabinose residue from DPA in an α(1→3)-linkage to an arabinan acceptor e.g. NV1. The extended product then serves as a precursor for subsequent extension by a β(1→2)-arabinosyltransferase catalyzed by AftB, resulting in the synthesis of the terminal branching hexaarabinofuranosyl motif found in AG. DPA, decaprenyl-phosphate-arabinose; DP, decaprenyl-phosphate; AG, arabinogalactan.
(B) Schematic representation of the α(1→5) arabinosyltransferase reaction catalyzed by EmbC2-AcpM2complex, leading to elongation of the arabinan chain in the LAM precursor. The structure of LAM precursor may contain several mannose groups and arabinose groups, simplified here for clarity. LAM, lipoarabinomannan.
(C) (up) The designed reaction scheme illustrating α(1→3) arabinosyltransferase (EmbA-EmbB) and β(1→2) arabinosyltransferase (AftB) activity assays. (down) Cell-free α(1→3)-arabinosyltransferase activity of the purified wild-type EmbA-EmbB complexes and catalytic site mutations in the presence and absence of ethambutol (see also fig. S1J). NV1 was used as the acceptor and DP[14C]A as the donor as described in previous studies (11, 12, 16). Msm membrane contains ethambutol-resistant arabinosyltransferase AftB. EMB, ethambutol. Data presented are the mean values +SD calculated from three independent experiments.
(D) (up) The designed reaction scheme illustrating an α(1→5) arabinosyltransferase activity assay to characterize EmbC2 activity. (down) Cell-free α(1→5)-arabinosyltransferase activity of the purified EmbC2 complex in the presence and absence of ethambutol (see also fig. S2G). NV6 was used as the acceptor and DP[14C]A as the donor as described in previous studies (13, 16). Msm membrane contains ethambutol-resistant arabinosyltransferase AftB. EMB, ethambutol. Data presented are the mean values +SD calculated from three independent experiments.
(E) Overall view of cryo-EM structures of Mtb and MsmEmbA-EmbB-AcpM2 complexes in complex with ethambutol or di-arabinose, and cryo-EM and crystal structures of Msm EmbC2-AcpM2complexes in complex with ethambutol or di-arabinose. The AcpM protomer binds to each Emb protein in all the complexes, un-modeled AcpM protomer bound to Mtb EmbB or Msm EmbA are colored in gray. The drug EMB, the substrate DPA and Ara2, the lipids are shown as spheres. Ara2, di-arabinose.
(F) The overall fold of Emb proteins represented by Mtb EmbB. The PN, PC and TM domains are colored differently. The functional important PL2 and PL5 are also highlighted. The location of active site is marked by a dashed circle. PN/PC, N-/C-terminal periplasmic domain; TM transmembrane domain; PL, periplasmic loop connecting two transmembrane helices.
(G) Topological diagram of Emb proteins colored as in (F), DDx motif is shown as three red spheres. CL, cytoplasmic loop connecting two transmembrane helices; EH, extra-cellular helix.
