29 Nov 2018
Abstract
Iron’s abundance and rich coordination chemistry are potentially appealing features for photochemical applications. However, the photoexcitable charge-transfer (CT) states of most Fe complexes are limited by picosecond or sub-picosecond deactivation through low-lying metal centered (MC) states, resulting in inefficient electron transfer reactivity and complete lack of photoluminescence. Here we show that octahedral coordination of Fe(III) by two mono-anionic facial tris-carbene ligands can suppress such deactivation dramatically. The resulting complex [Fe(phtmeimb)2]+, where phtmeimb is [phenyl(tris(3-methylimidazol-1-ylidene))borate]-, exhibits strong, visible, room temperature photoluminescence with a 2.0 ns lifetime and 2% quantum yield via spin-allowed transition from a ligand-to-metal charge-transfer (2LMCT) state to the ground state (2GS). Reductive and oxidative electron transfer reactions were observed for the 2LMCT state of [Fe(phtmeimb)2]+ in bimolecular quenching studies with methylviologen and diphenylamine.
[Image]
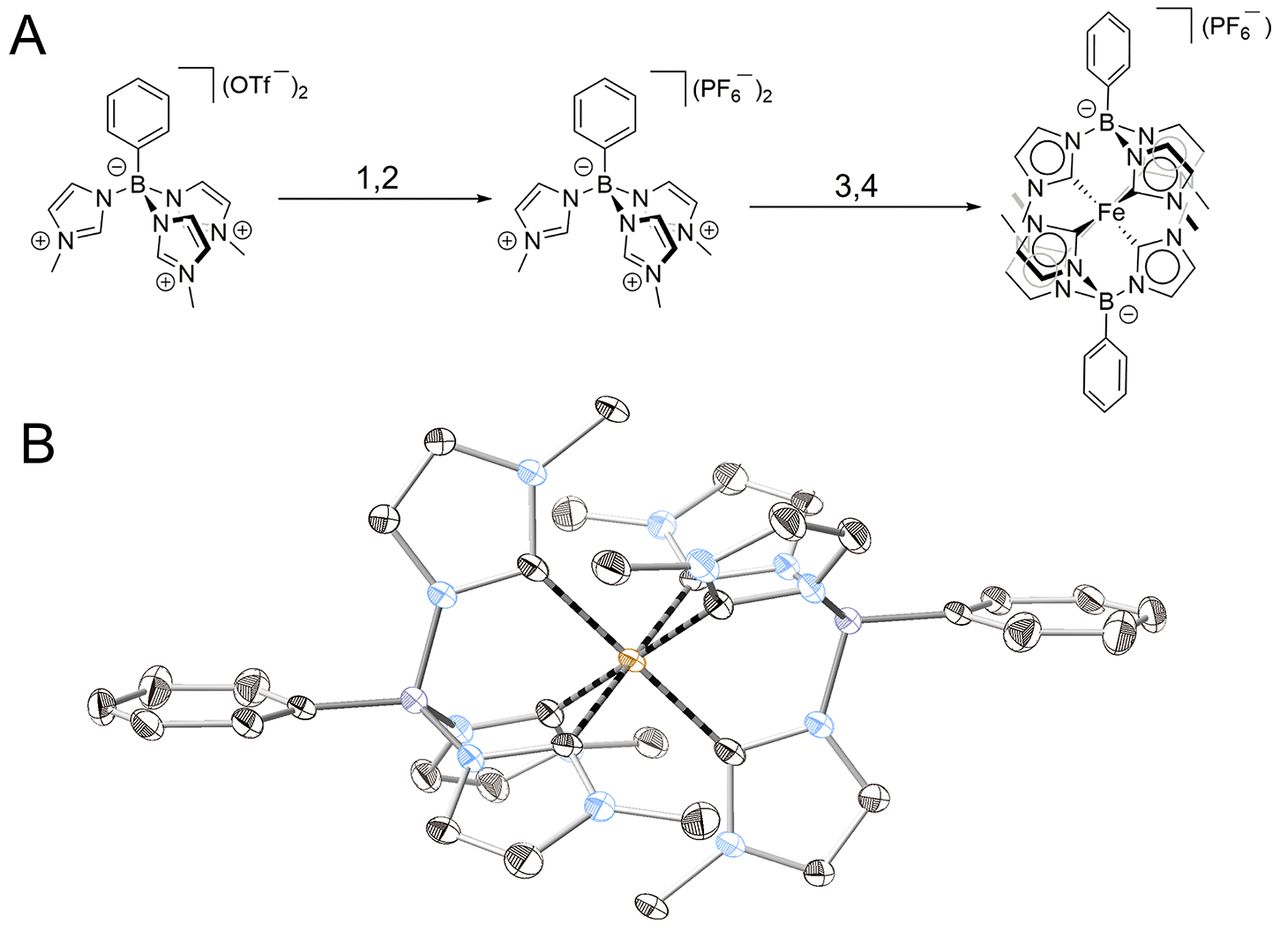
Fig. 1 Synthesis and structure of [Fe(phtmeimb)2]PF6.
(A) Synthetic route: 1, precipitation with tetra-n-butyl-ammonium bromide in acetone; 2, dissolution in water and precipitation with ammonium hexafluorophosphate; 3, dissolution in tetrahydrofuran under N2, cooling to –78°C and addition of tert-butoxide; 4, addition of FeBr2, stirring under N2 at room temperature for 24 hours.
(B) X-ray crystal structure. Thermal ellipsoids are shown at 50% probability with the six Fe-C bonds highlighted in black. Hydrogen atoms, counter ions and solvent molecules are omitted for clarity. Fe, orange; B, purple; N, blue; C, black.
